

Dowload our webinar to learn more about Substance Information Management.
For many pharmaceutical companies, there is a daunting task ahead to align and maintain substance data throughout the company. During this webinar we will present our experience on substance management, and we share the challenges we have seen in various companies.
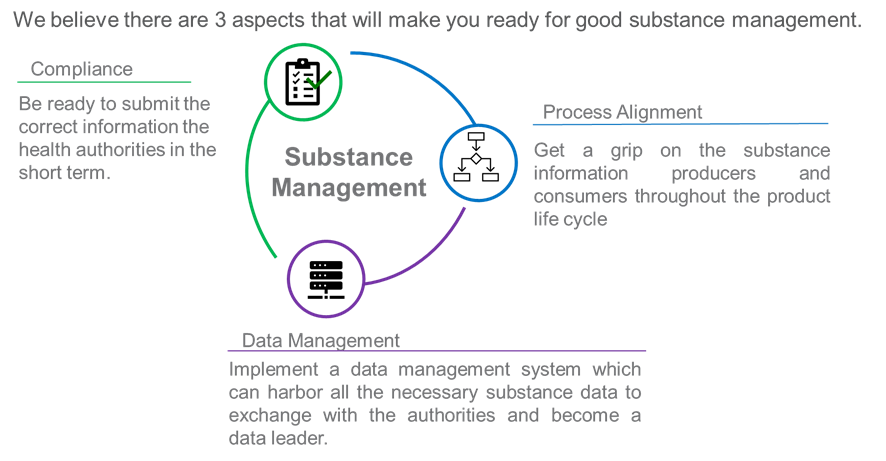
During this webinar you will learn
 |
| Frank Geelen Partner Deloitte Nederland CFO Programma T +31882884659 E fgeelen@deloitte.nl |


|
| Frank Geelen Partner Deloitte Nederland CFO Programma T +31882884659 E fgeelen@deloitte.nl |


|
| Frank Geelen Partner Deloitte Nederland CFO Programma T +31882884659 E fgeelen@deloitte.nl |


|
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Curabitur luctus convallis quam vitae finibus. Aliquam quis congue turpis, nec euismod sem. Duis rutrum mi sit amet risus interdum, et sollicitudin nisl dictum. Sed eu libero sapien. Aliquam magna ligula, lobortis in neque et, commodo faucibus lorem. Donec vitae venenatis purus. Donec mollis dui vel suscipit imperdiet. Vestibulum id nibh non magna dignissim pharetra ac luctus lorem.